CU, Silpakorn Sign MoU to Develop Anti-Covid Nasal Spray Before Yearend
Two universities signed a memorandum of understanding (MoU) with three partners on Monday to develop an anti-Covid-19 nasal spray.
he MoU was signed by Prof Dr Chanchai Sittipunt, dean of Chulalongkorn University’s Faculty of Medicine, Silpakorn University rector Chaicharn Thavaravej, Dr Nopporn Chuenklin, director of the Health Systems Research Institute, Withoon Danwiboon, director of Government Pharmaceutical Organisation (GPO), and Cdr Dr Phaporn Prasitdamrong, an executive of Hibiocy Co Ltd.
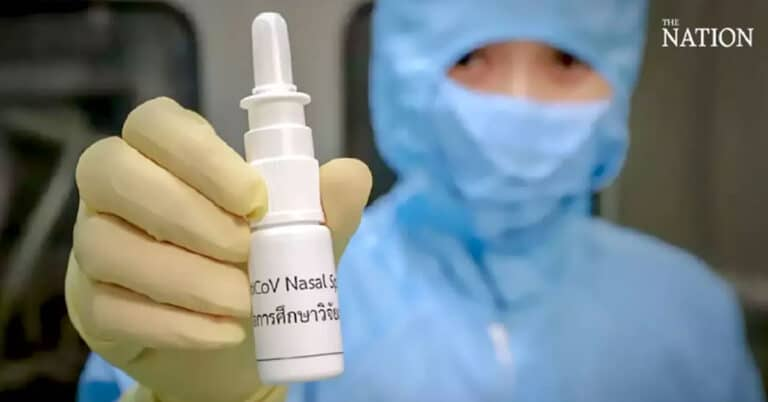
The aim of the MoU is to develop an anti-SAR-CoV-2 virus nasal spray that can be widely sold both locally and internationally.
The spray has been successfully tested on animals and a clinical trial on volunteers is being planned for the first quarter of this year. If this trial proves to be successful, the spray should be registered with the Thai Food and Drug Administration by June and go into mass production within the third quarter. thailand universities
Getting ready for launch
Chanchai said the spray, part of a long list of anti-Covid products developed by Chulalongkorn University, was developed with support from the Health Systems Research Institute. He added that the university has patented the antibodies created to make the spray and that it is now ready to work with other institutions to get the product ready for clinical testing.
Meanwhile, Silapakorn University’s rector Chaicharn said researchers from his university have been working closely with their counterparts in Chulalongkorn to develop a prototype for the spray. He said animal testing shows the spray can effectively prevent infection, so he hopes his five partners will soon be able to produce a product that the public can use to survive the Covid-19 crisis.
Dr Nopporn from the Health Systems Research Institute said the development of the nasal spray is an addition to the research on monoclonal antibody cocktail against Covid-19 that his institute has helped Chulalongkorn researchers to develop.
Withoon, meanwhile, said the GPO will help produce nasal sprays for clinical testing among volunteers before the product is registered with the FDA.
Phaporn said her company, Hibiocy, will later help distribute the spray both locally and internationally.
The spray will be an alternative to Covid-19 vaccines for preventing infections because it will stop the virus from entering the body via the nasal passage, she said, adding that it should be ready to go on the shelves by the third quarter of this year.
ความคิดเห็น
แสดงความคิดเห็น